Introduction
Enzymes are globular proteins, responsible for most of the chemical activities of living organisms. They act as catalysts, substances that speed up chemical reactions without being destroyed or altered during the process. Enzymes are extremely efficient and may be used over and over again. One enzyme may catalyze thousands of reactions every second.
H2O2 is toxic to most living organisms. Many organisms are capable of enzymatically destroying the H2O2 before it can do much damage. H2O2 can be converted to oxygen and water, as follows:
Although this reaction occurs spontaneously, enzymes increase the rate considerably. Catalase, a common enzyme that is found in the cells of nearly all living organisms, catalyzes the decomposition of H2O2. A great deal can be learned about enzymes by studying the rates of enzyme-catalyzed reactions.
Objectives
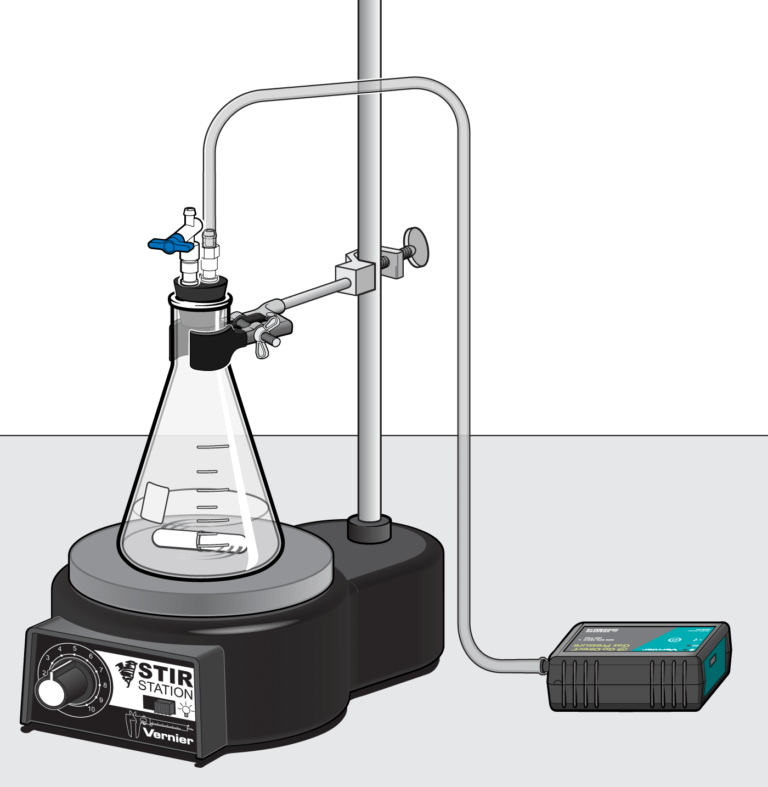
In the Preliminary Activity, you will use catalase in yeast to catalytically decompose hydrogen peroxide. You will determine the rate of enzyme activity by measuring the pressure of oxygen gas produced as H2O2 is decomposed.
After completing the Preliminary Activity, you will first use reference sources to find out more about enzymes and enzyme activity before you choose and investigate a researchable question dealing with enzyme activity.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.
Correlations
Teaching to an educational standard? This experiment supports the standards below.
- International Baccalaureate (IB) 2025/Chemistry
- Reactivity 2.2.3—Factors that influence the rate of a reaction include pressure, concentration, surface area, temperature and the presence of a catalyst.
Ready to Experiment?
Ask an Expert
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email support@vernier.com
Purchase the Lab Book
This experiment is #23 of Investigating Chemistry through Inquiry. The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.