Biochemistry
Help Your Students Understand the Structure of Living Systems
Our integrated solution helps students collect accurate data, visualize trends and relationships, and explore different hypotheses for both conventional and innovative experiments.
QUALITY
For years, colleges and universities have relied on our durable hardware to help instructors teach key concepts.
AFFORDABLE
Our technology is designed and priced for student use.
VERSATILE
Biochemistry Product Categories
Chemistry Resources
Example Data
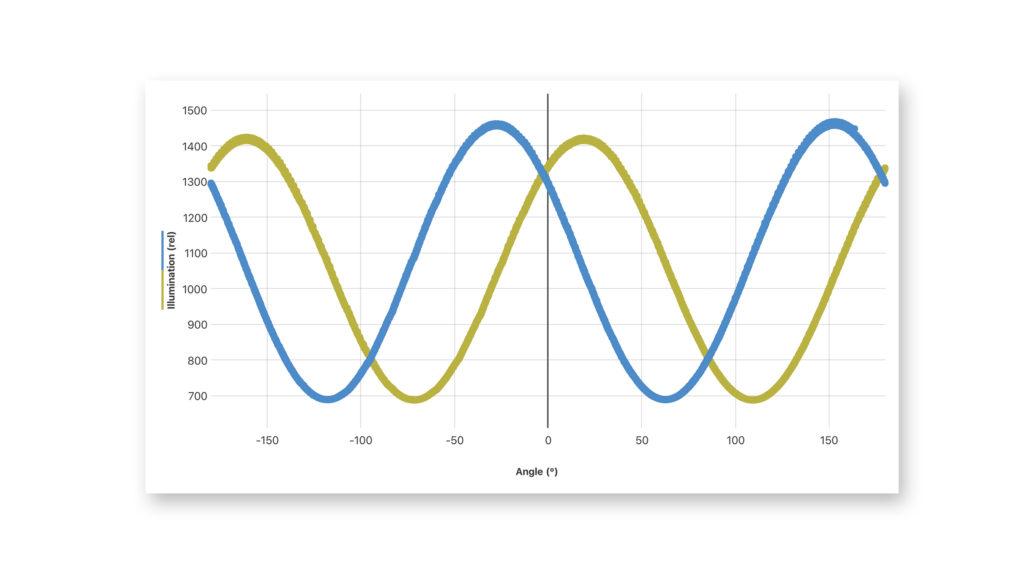
Isolating and characterizing protein and sugar from milk with the Go Direct® Polarimeter and Vernier Instrumental Analysis™
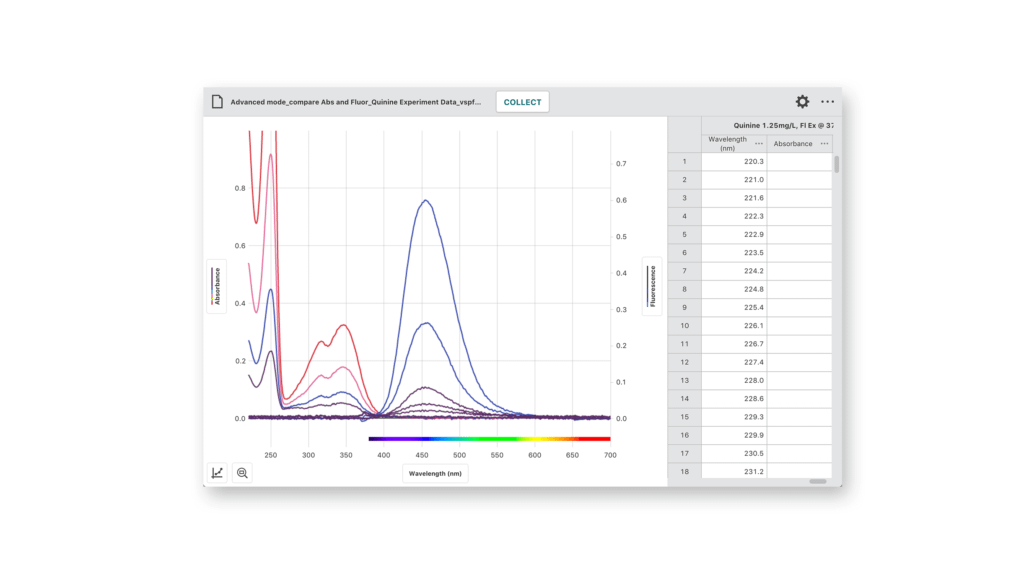
Examining the absorbance and fluorescence spectra of quinine sulfate at varying concentrations with the Vernier Fluorescence/UV-VIS Spectrophotometer and Vernier Spectral Analysis®
This is only the beginning of what’s possible. See the recommendations below to get started with biochemistry.
Free Experiment Downloads
Featured Biochemistry Experiments
Featured Biochemistry Products
-
Vernier Graphical Analysis® Pro
Start a Free Trial -
Go Direct® O2 Gas Sensor
$219.00 -
Go Direct® Polarimeter
$545.00 -
Go Direct® Drop Counter
$115.00 -
Stir Station
$149.00 -
Electrode Support
$10.00 -
Blue Digital Bioimaging System
$799.00
This product has been discontinued.