Ammonium Ion-Selective Electrode User Manual
Order Code: NH4-BTA
The Vernier Ammonium Ion-Selective Electrode is used to measure the concentration of ammonium (NH4+) ions in aqueous samples.
Note: Vernier products are designed for educational use. Our products are not designed nor are they recommended for any industrial, medical, or commercial process such as life support, patient diagnosis, control of a manufacturing process, or industrial testing of any kind.
What's Included
- Ammonium Ion-Selective Electrode, packed in a storage bottle
- 30 mL bottle of High Standard solution with SDS (100 mg/L NH4+ as N)
- 30 mL bottle of Low Standard solution with SDS (1 mg/L NH4+ as N)
- Short-Term ISE Soaking Bottle
Compatible Software
Choose a platform below to see its compatibility requirements.LabQuest
Interface LabQuest App LabQuest 3 Full support LabQuest 2 Full support LabQuest Full support Computers
Software Interface Graphical Analysis Graphical Analysis (Web App) Logger Pro (discontinued) Logger Lite (discontinued) LabQuest Mini Full support Full support Full support Full support LabQuest 3 Full support Full support Full support Incompatible LabQuest 2 Full support Full support Full support Full support LabQuest Stream Full support 1 Full support 1 Partial support 2 Full support 1 Go!Link Full support Full support Full support Full support LabQuest Full support Full support Full support Full support LabPro Incompatible Incompatible Full support Full support Compatibility Notes
Chromebook
Software Interface Graphical Analysis (Web App) LabQuest Mini Full support LabQuest 3 Full support LabQuest 2 Full support LabQuest Stream Full support 1 Go!Link Full support LabQuest Full support Compatibility Notes
iOS
Software Interface Graphical Analysis Graphical Analysis GW LabQuest Stream Full support Full support LabQuest 3 Full support 1 Full support 1 LabQuest 2 Full support 1 Full support 1 Compatibility Notes
Android
Software Interface Graphical Analysis Graphical Analysis GW LabQuest Stream Full support Incompatible LabQuest 3 Full support 1 Full support 1 LabQuest 2 Full support 1 Full support 1 Compatibility Notes
Arduino
Software Interface Arduino Vernier Arduino® Interface Shield Full support 1 Compatibility Notes
LabVIEW
Software Interface NI LabVIEW SensorDAQ Full support 1 Vernier myDAQ Adapter Full support 1 2 Go!Link Full support 1 LabQuest Mini Full support LabQuest Stream Full support LabQuest 3 Full support LabQuest 2 Full support LabQuest Full support Compatibility Notes
Texas Instruments
Software Interface EasyData DataMate TI-84 SmartView DataQuest TI-Nspire Software EasyLink Full support 1 Incompatible Full support 2 Full support Full support 2 CBL 2 Full support 3 Full support 3 4 Incompatible Incompatible Incompatible LabPro Full support 3 Full support 3 4 Incompatible Incompatible Incompatible TI-Nspire Lab Cradle Incompatible Incompatible Incompatible Full support Full support Compatibility Notes
Quick Start
- Prepare the electrode by soaking it in the High Standard solution for 30 minutes. Refer to the next section for more information.
- Plug the sensor into the interface (LabQuest 3, LabQuest Mini, etc.).
- Connect the interface to your device.
- If using USB, connect to the USB port on your computer.
- If using Bluetooth® wireless technology, click your interface type and then select your device.
- Prepare for data collection:
- Vernier Graphical Analysis®: Launch the app, if necessary, and click Sensor Data Collection.
- LabQuest® App: Choose New from the File menu.
- The software will identify the sensor and load a default data-collection setup. You are now ready to collect data.
- Perform a two-point calibration using the High and Low Standard solutions. Refer to the next section for more information.
Need Additional Information?
Visit the following link:
Preparing the Ammonium ISE for Use
Note: Follow this two-part process before taking measurements with your ISE.
Part I: Soak the Electrode
Soak the electrode in the High Standard solution (included with the ISE) for approximately 30 minutes. The ISE should not rest on the bottom of the container, and the small white reference contacts near the tip of the electrode should be immersed. Make sure no air bubbles are trapped below the ISE. Important: Do not leave the ISE soaking for more than 24 hours. Important: If you plan to use the electrode outside the range of the standards provided, you will need to prepare your own standards and use those for soaking.
Note: If the ISE needs to be transported to the field during the soaking process, use the Short-Term ISE Soaking Bottle. Remove the cap from the bottle and fill it 3/4 full with High Standard. Slide the bottle’s cap onto the ISE, insert it into the bottle, and tighten.
For long term storage, greater than 24 hours, make sure the sensor is stored in its storage bottle with the sponge slightly damp.
Part II: Calibrate the ISE
Calibrating the Ammonium ISE in Graphical Analysis
- Connect the sensor according to the Getting Started section.
- Click or tap the live readouts meter and choose Calibrate.
- High Standard Calibration Point: The Ammonium ISE should still be soaking in the High Standard. The ISE should not rest on the bottom of the container, and the 2 small white reference contacts near the tip of the electrode should be immersed. Make sure no air bubbles are trapped below the ISE.
- Enter the concentration value of the High Standard (e.g., 100 for 100 mg/L) in the edit box and click or tap Keep.
- Low Standard Calibration Point: Remove the ISE from the High Standard, rinse well with distilled water, and gently blot the ISE dry with a paper towel. Place the ISE into the Low Standard. Make sure the ISE is not resting on the bottom of the container, the white reference contacts near the tip of the electrode are immersed, and no air bubbles are trapped below the ISE.
- Enter the concentration value for the Low Standard (e.g., 1 for 1 mg/L) and click or tap Keep .
- Click or tap Apply to complete the calibration process.
Calibrating the Ammonium ISE with LabQuest App
- Connect the sensor according to the Getting Started section.
- High Standard Calibration Point: The Ammonium ISE should still be soaking in the High Standard. The ISE should not rest on the bottom of the container, and the small white reference contacts near the tip of the electrode should be immersed. Make sure no air bubbles are trapped below the ISE.
- Enter the concentration of the High Standard (e.g., 100 for 100 mg/L) for Reading 1.
- After the voltage reading stabilizes (~2 minutes), tap Keep.
- Low Standard Calibration Point: Remove the ISE from the High Standard, rinse well with distilled water, and gently blot the ISE dry with a paper towel. Place the ISE into the Low Standard. Make sure the ISE is not resting on the bottom of the container, the white reference contacts near the tip of the electrode are immersed, and no air bubbles are trapped below the ISE.
- Enter the concentration of the Low Standard (e.g., 1 for 1 mg/L) for
Reading 2. - After the voltage reading stabilizes, tap Keep.
- To save the calibration to the sensor, follow the steps below:
- Tap Storage.
- Tap Save Calibration to Sensor. Tap OK.
- Tap OK to complete the process.
Using the Product
The Ammonium Ion-Selective Electrode (ISE) can be used to determine concentrations of NH4+ ions in aqueous solutions in units of mg/L. Concentrations of aqueous ammonium ions should not be mistaken for concentration of aqueous ammonia, or NH3(aq). The concentrations of these two species, though different, are often involved in the same equilibrium reaction:
Reaction 1: NH3(aq) + H+(aq) ↔ NH4+ (aq)
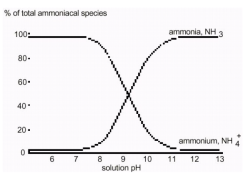
In a more acidic environment, higher concentrations of H+ ions will cause this reaction to shift toward the right, resulting in higher concentrations of NH4+. In a more basic (alkaline) environment, the concentration of NH4+ will be lower, causing the reaction to shift toward the reactants, producing higher concentrations of NH3. At pH values greater than 10 (see Figure 1), most of the ammonium ions will be converted to ammonia. At pH values less than 7.5, most of the aqueous ammonia will be converted to ammonium ions.
Sampling Freshwater Samples for Ammonium Concentration
While permissible levels of ammonium in drinking water should not exceed 0.5 mg/L, streams or ponds near heavily fertilized fields may have higher concentrations of this ion. Fertilizers containing ammonium sulfate, (NH4)2SO4, or ammonium nitrate, NH4NO3, may result in runoff from fields containing higher levels of the ammonium ion, NH4+. Monitoring ammonium levels on a stream that borders fertilized fields may show significant seasonal differences in NH4+ concentrations. In this kind of study, you may also take pH measurements in your water samples; as indicated in the previous paragraph, higher or lower pH values can greatly affect the ratio of NH4+ / NH3 in a sample. Since the Ammonium ISE measures only NH4+ levels, you may want to adjust your samples to the same pH value each time you make measurements; this may not be necessary if you have relatively “hard” water. Hard water is naturally buffered against changes in pH.
Expressing Ammonium Concentration
Concentrations of ammonium are often expressed in units of mg/L NH4+ as N. Here is a calculation for a 100 mg/L NH4+ as N standard solution that is prepared by adding solid NH4Cl to distilled water:
How Can I Have My ISE Read mV Output Instead of mg/L?
The mV output of the electrode can be calculated using the following equation:
-23.889*(-7.487 – ln(“Ammonium (mg/L)”)) – 168.23 = Ammonium (mV)
This equation provides the mV output of the electrode, before amplification. If you want post-amplification Volts, the equation is
V = 0.00727*mV + 1.223
Collecting Data
- Make sure the sensor is properly calibrated. If the meter has a reading of 1.0 mg/L and the sensor is not in a 1.0 mg/L solution, you need to calibrate. After calibration, rinse off the tip of the ISE and blot it dry with a paper towel.
- Insert the tip of the ISE into the aqueous sample to be tested. Important: Make sure the ISE is not resting on the bottom of the container, the white reference contacts near the tip of the electrode are immersed, and no air bubbles are trapped below the ISE. Note: Do not completely submerge the sensor. The handle is not waterproof.
- Hold the ISE still until the reading stabilizes and record the displayed reading. Note: With some aqueous samples, especially those at high concentrations, it could take several minutes for the reading of the Ammonium ISE to stabilize. If you know the approximate concentrations of your samples, it is best to analyze them from lowest concentration to highest.
Using the Ammonium ISE with Other Vernier Sensors
Some combinations of sensors interfere with each other when placed in the same solution. The degree of interference depends on many factors. For more information, see www.vernier.com/til/638/
Using Ionic Strength Adjustor (ISA) Solutions to Improve Accuracy
For optimal results at low concentrations of ions, a standard method for making measurements with the Ammonium Ion-Selective Electrode (ISE) is to add ionic strength adjustor (ISA) solutions to each of your standard solutions and samples.
Adding an ISA ensures that the total ion activity in each solution being measured is nearly equal, regardless of the specific ion concentration. This is especially important when measuring very low concentrations of specific ions. The ISA contains no ions common to the ISE itself. Note: The addition of ISA to samples or standards does not need to be highly accurate. You can add the ISA solution dropwise to a sample using a disposable plastic Beral pipet. We recommend using 0.25 M magnesium acetate solution prepared in 0.5 M acetic acid solution as the ISA for the Ammonium ISE. To prepare this solution, dissolve 53.6 grams of magnesium acetate in sufficient 0.5 M acetic acid solution to make 1.0 liter. Commonly, ISA is added in a 1:50 ratio, or 1 mL of ISA added to 50 mL of water to be tested.
Videos
Specifications
|
Range |
1 to 18,000 mg/L (or ppm) |
|
Reproducibility (precision) |
±10% of full scale (calibrated 1 to 100 mg/L) |
|
Interfering ions |
K+, Li+, Na+, Cs+, Mg3+, Ca2+, Sr2+, Ba2+ |
|
pH range |
4–7.5 (no pH compensation) |
|
Temperature range |
0–40°C (no temperature compensation) |
|
Electrode slope |
+56 ±4 mV/decade at 25°C |
|
Electrode resistance |
1 to 4 MΩ |
|
Immersion |
2.8 cm |
|
Electrode length |
155 mm |
|
Body diameter |
12 mm |
|
Cap diameter |
16 mm |
|
Cable length |
100 cm |
|
Calibration voltages, typical |
High (100 mg/L) 2.1 V, Low 1.3 V (1 mg/L) |
Care and Maintenance
Storing the Ion-Selective Electrode
Proper care and storage are important for optimal longevity of your Ammonium ISE.
- Long-term storage of the ISE (longer than 24 hours): Moisten the sponge in the bottom of the long-term storage bottle with distilled water. When you finish using the ISE, rinse it off with distilled water and blot it dry with a paper towel. Loosen the lid of the long-term storage bottle and insert the ISE. Note: The tip of the ISE should NOT touch the sponge. Also, make sure the white reference mark is inside the bottle. Tighten the lid. This will keep the electrode in a humid environment, which prevents the reference junctions from completely drying out.
- Short-term wet storage (less than 24 hours): Fill the Short-Term ISE Soaking bottle 3/4 full with High Standard. Loosen the cap, insert the electrode into the bottle, and tighten.
Maintaining and Replacing the ISE Standard Calibration Solutions
Having accurate standard solutions is essential for performing good calibrations. The two standard solutions that were included with your ISE can last a long time if you take care not to contaminate them. At some point, you will need to replenish your supply of standard solutions. Vernier sells replacement standards in 500 mL volumes. Order codes are:
- NH4-LST: Ammonium Low Standard, 1 mg/L
- NH4-HST: Ammonium High Standard, 100 mg/L
To prepare your own standard solutions, use the information in the table below. Note: Use glassware designed for accurate volume measurements, such as volumetric flasks or graduated cylinders. All glassware must be very clean.
| Standard Solution | Concentration (mg/L or ppm) | Preparation Method using High-Quality Distilled Water |
|
Ammonium (NH4+) ISE High Standard |
100 mg/L NH4 as N |
0.382 g NH4Cl/ 1 L solution |
|
Ammonium (NH4) ISE Low Standard |
1 mg/L NH4 as N |
Dilute the High Standard by a factor of 100 (from 100 mg/L to 1 mg/L) |
Do not wrap the cable tightly around the sensor for storage. Repeatedly doing so can irreparably damage the wires and is not covered under warranty.
How the Sensor Works
The Vernier Ammonium Ion-Selective Electrode (ISE) is a membrane-based electrode that measures a specific ion (NH4+) in an aqueous solution. When the membrane of the electrode is in contact with a solution containing the specific ion, a voltage, dependent on the level of that ion in solution, develops at the membrane. The ISE is a combination style electrode. The voltage develops in relation to an internal Ag/AgCl reference electrode. The ISE measures for the specific ion concentration directly. Samples need to be aqueous to avoid contaminating or dissolving the membrane. The Vernier Ammonium Ion-Selective Electrode has a solid polymer membrane. The membrane is a porous plastic disk, permeable to the ion exchanger, but impermeable to water. It allows the sensing cell to contact the sample solution and separates the internal filling solution from the sample.
The voltage developed between the sensing and reference electrodes is a measure of the concentration of the reactive ion being measured. As the concentration of the ion reacting at the sensing electrode varies, so does the voltage measured between the two electrodes.
As described in the Nernst Equation, ISE response is a linear equation:
E = Eo + m(ln a)
where E is the measured voltage, Eo is the standard potential for the combination of the two half cells, m is the slope, ln is the natural logarithm, and a is the activity of the measured ion species.
Assuming the ionic strength is fairly constant, the Nernst equation may be rewritten to describe the electrode response to the concentration, C, of the measured ion species:
E = Eo + m(ln C)
Troubleshooting
For troubleshooting and FAQs, see www.vernier.com/til/1435
Repair Information
If you have watched the related product video(s), followed the troubleshooting steps, and are still having trouble with your Ammonium Ion-Selective Electrode, contact Vernier Technical Support at support@vernier.com or call 888-837-6437. Support specialists will work with you to determine if the unit needs to be sent in for repair. At that time, a Return Merchandise Authorization (RMA) number will be issued and instructions will be communicated on how to return the unit for repair.
Accessories/Replacements
Ammonium ISE Replacement Membrane Modules
The Ammonium ISE has a PVC membrane module with a limited life expectancy. The module is warranted to be free from defects for a period of twelve (12) months from the date of purchase. It is possible, however, that a membrane module will work well after the warranty period. If you notice a reduced response (e.g., distinctly different voltages or voltage ranges during calibration), then it is probably time to replace the membrane module. Important: Do not order membrane modules far in advance; the process of degradation takes place even when the modules are stored on the shelf.
Additional Vernier Ion-Selective Electrodes
Vernier sells Ion-Selective Electrodes that measure the concentration of calcium (Ca2+), chloride (Cl–), nitrate (NO3–), and potassium (K+) ions in aqueous solutions:
| Item | Order Code |
|---|---|
|
CA-BTA |
|
| Chloride Ion-Selective Electrode |
CL-BTA |
|
NO3-BTA |
|
| Potassium Ion-Selective Electrode |
K-BTA |
| Electrode Storage Bottles, pkg of 5 |
BTL-ES |
| Standard High Ammonium ISE Solution |
NH4-HST |
| Standard Low Ammonium ISE Solution |
NH4-LST |
| Ammonium Replacement Module |
NH4-MOD |
Warranty
Warranty information for this product can be found on the Support tab at www.vernier.com/nh4-bta/#support
General warranty information can be found at www.vernier.com/warranty
Disposal
When disposing of this electronic product, do not treat it as household waste. Its disposal is subject to regulations that vary by country and region. This item should be given to an applicable collection point for the recycling of electrical and electronic equipment. By ensuring that this product is disposed of correctly, you help prevent potential negative consequences on human health or on the environment. The recycling of materials will help to conserve natural resources. For more detailed information about recycling this product, contact your local city office or your disposal service.
Battery recycling information is available at www.call2recycle.org
 The symbol, shown here, indicates that this product must not be disposed of in a standard waste container.
The symbol, shown here, indicates that this product must not be disposed of in a standard waste container.
Contact Support
Fill out our online support form or call us toll-free at 1-888-837-6437.

