Bring Your Biology
Lessons to Life
Biology
Teach the fundamentals of biology, including cell respiration, photosynthesis, diffusion, osmosis, fermentation, and more, with Vernier technology.
Ready to get started with Go Direct?
Learn how to easily integrate Go Direct sensors into your classroom or lab.
Human Physiology
With Vernier technology, students can collect data for measuring heart rate, EKG, blood pressure, and lung function. Whether your students are aspiring health professionals or budding biomedical engineers, they will gain a deeper understanding of human physiology through our hands-on solutions.
Agricultural Science
Bring the world of plant, animal, and soil sciences alive for your students. With Vernier technology, students can build a foundational understanding of key agricultural science concepts such as soil quality, transpiration, and more.
Biotechnology
Bring cutting-edge technology to your biotech lab. Explore the properties of macromolecules, measure protein concentration, investigate biofuels, and more.
Environmental Science
Foster student engagement with the environment by using Vernier technology to investigate aquatic ecosystems, pollution, climate change, energy resources, and more.
New Products for Biology
Software for Biology
Engage Students in Three-Dimensional, Hands-On Learning
Vernier Connections deepens high school students’ understanding of scientific concepts and develops critical skills such as inquiry, problem solving, communication, and collaboration. Phenomenon-based, three-dimensional lessons combine the benefits of web‑based learning with hands-on investigation.

Collect, Graph, and Analyze Data in Real Time
Help students form critical connections between abstract scientific ideas and the real world. With the Vernier Graphical Analysis® app, students can visualize and interact with experiment data collected via nearly any Vernier sensor.
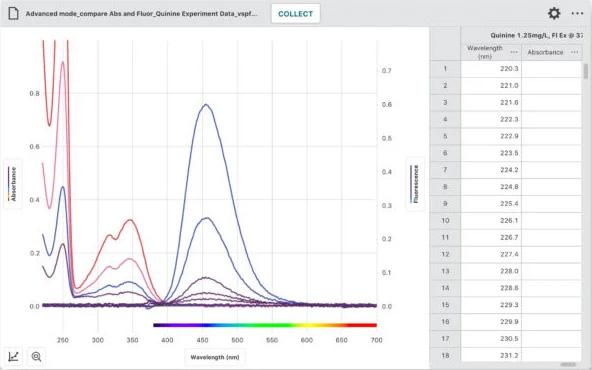
Capture, Analyze, and Share Spectrometer Data
Our free Vernier Spectral Analysis® app makes it easy to incorporate spectroscopy into your biology and chemistry labs. Using the app, students can collect a full spectrum and explore topics such as Beer’s law, enzyme kinetics, and plant pigments.

Lt by ADInstruments
Lt for Biology, developed in partnership with Vernier, covers biology concepts for college students including enzyme action, spectrophotometry, photosynthesis, and pH.
Vernier and Bio-Rad

Biology Product Categories
Biology Resources
We are scientists, educators, and your team.
As you implement data-collection technology into your teaching, we’re here to support you! Looking to learn more about our products or have questions about ordering? Reach out to our team at biology@vernier.com.
Are you a district or school administrator?
If you are considering an adoption or seeking training options, we are here to support you every step of the way. Contact our Science Technology Solutions Advisor, Candace Davis, at cdavis@vernier.com.